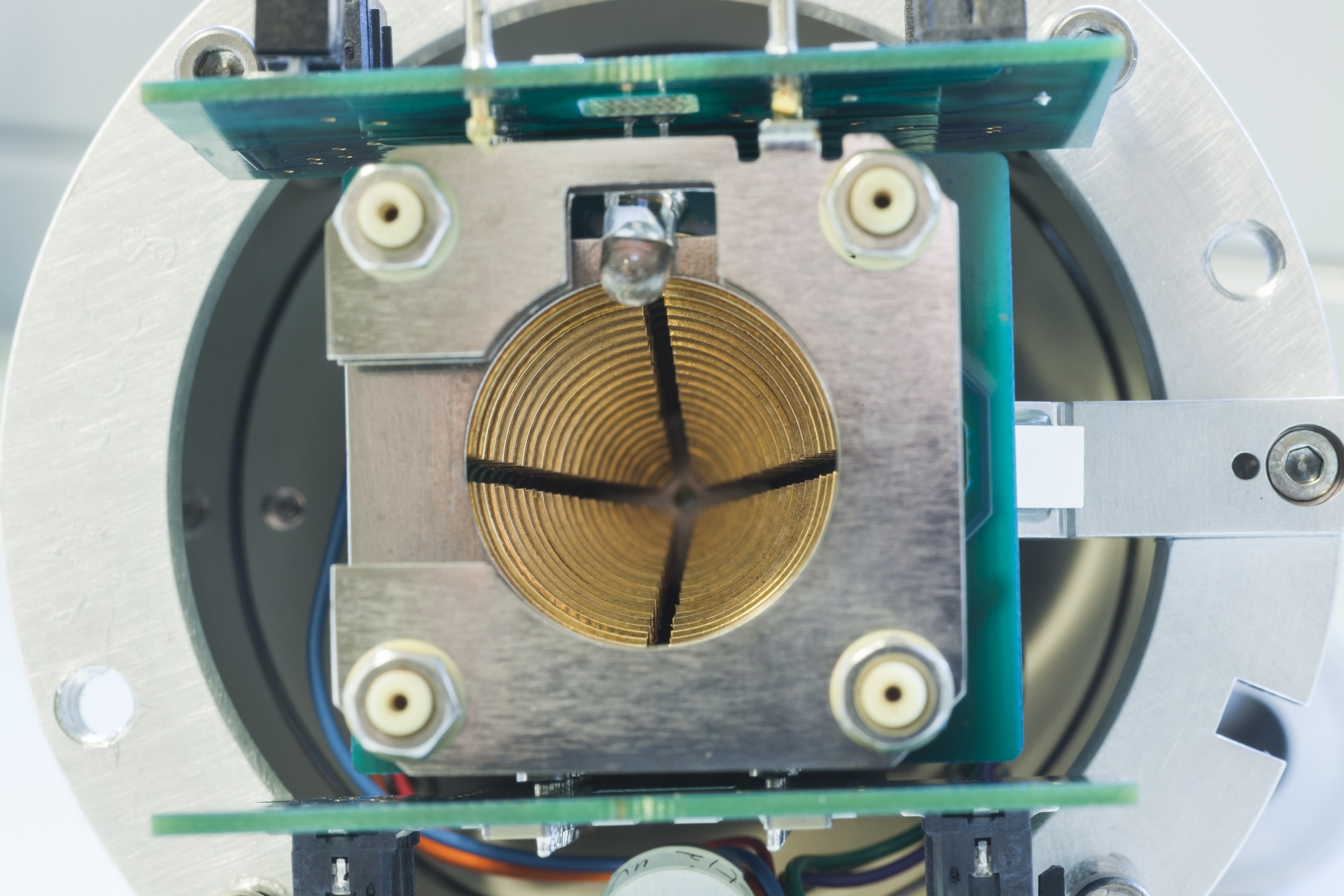
There are many ways to transform atoms or molecules into ions. Shooting energetic electrons at them belongs to the classic methods and is well known as electron ionization (EI). For EI to occur, it is important that the electrons possess more energy than required to eject an electron from the neutral species to be ionized (ionization energy) and that the neutrals are delivered in the gas phase.
In practice, compounds to be analyzed are evaporated into a high vacuum chamber (10–6 mbar) and exposed to electrons carrying a kinetic energy of 70 eV. These electrons are delivered by a brightly glowing tungsten (or rhenium) wire due the thermoelectric effect. They are extracted by a voltage of 70 V from the wire and accelerated. By the way, the same principle of electron beam generation is (essentially was) exploited in television tubes.
Obviously, a sequence of evaporation, ionization, and possibly subsequent fragmentation of these freshly generated ions implies that the sample is consumed during mass spectral analysis. Fortunately, mass spectrometry is an extremely sensitive method requiring less than 1 µg of sample for routine analyses and down to pg-amounts in trace analysis. Thus, sample consumption can be neglected in practice.

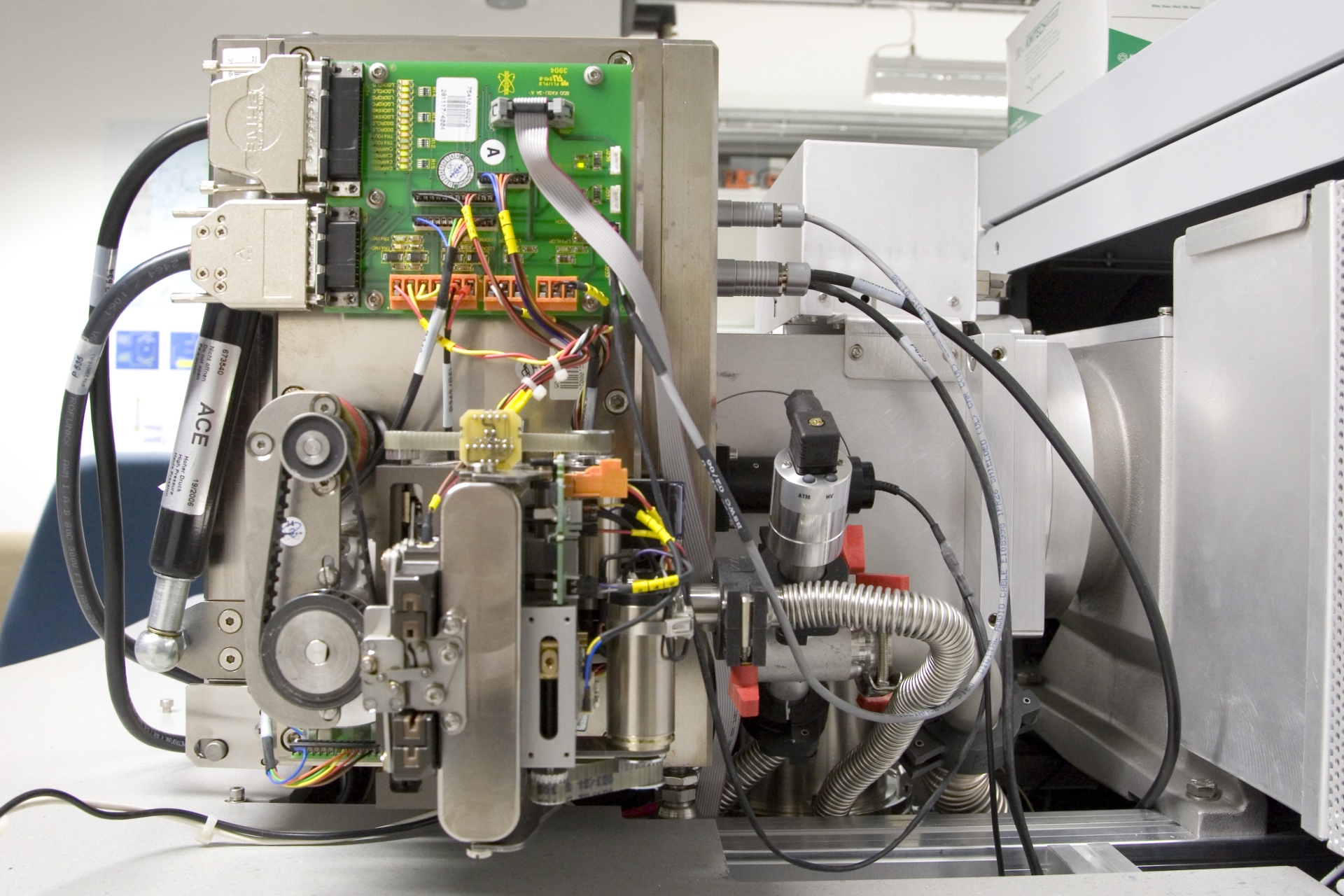
Fig. An ion source for electron ionization mounted on a magnetic sector mass spectrometer. Electric connections are on the right side, inlets for sample vapor from either a reservoir inlet or a gas chromatograph are on the left. The high vacuum pump is located directly below the ion source.