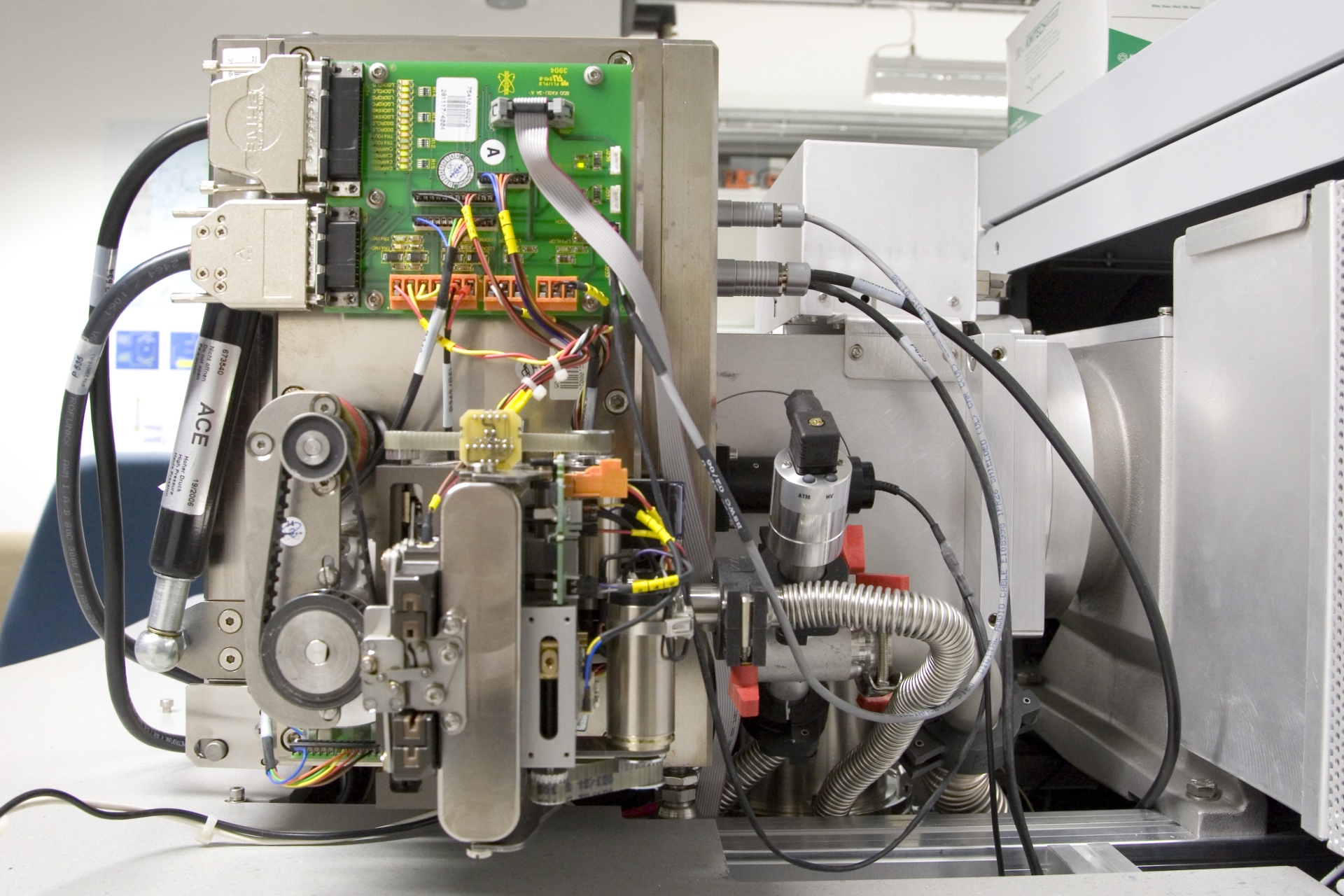
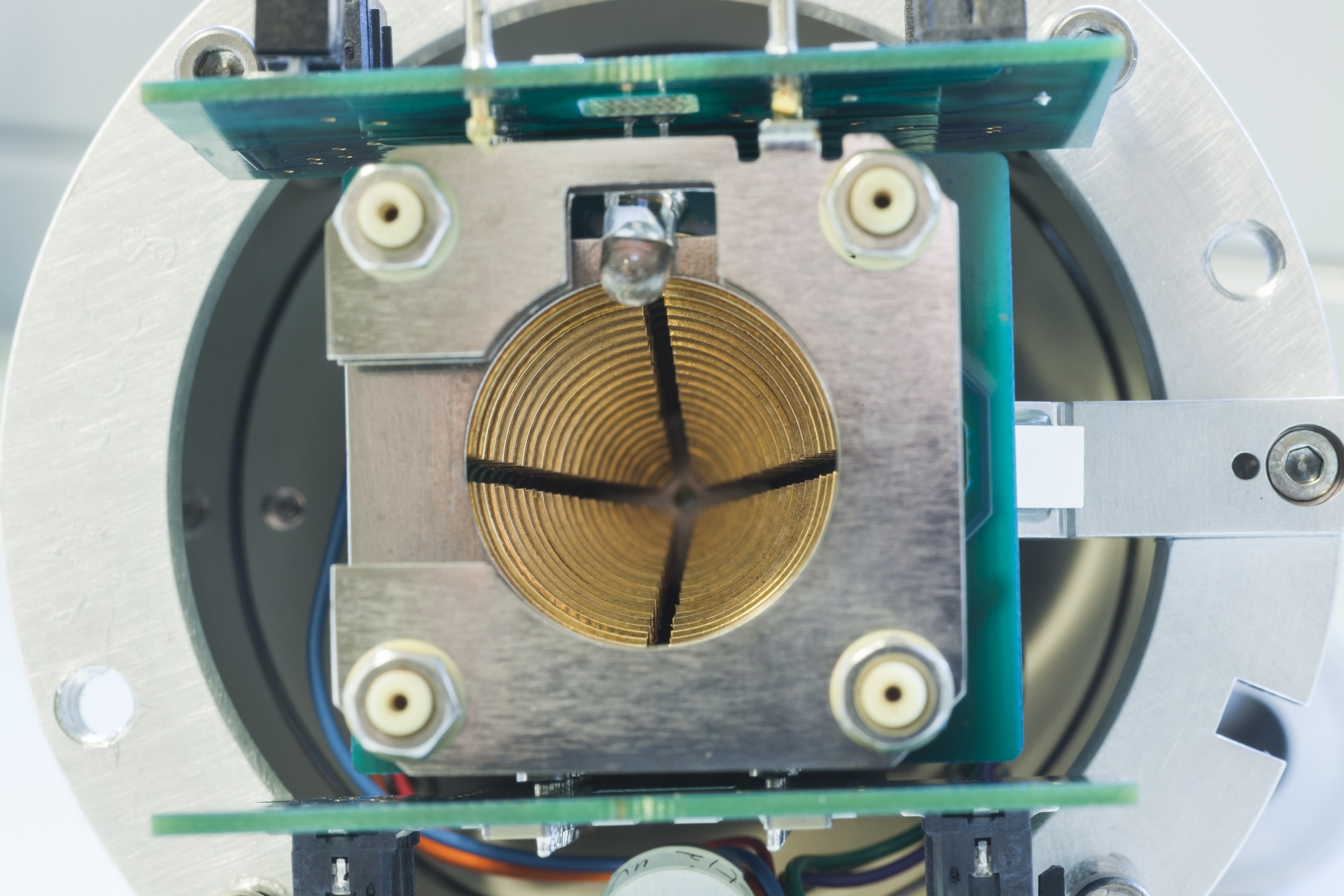
In mass spectrometry, there are numerous methods of ionizing the sample molecules. First, samples differ in volatility, i.e., some are gaseous, others are liquid or solid. Samples can be soluble in some solvent or insoluble, they may decompose upon contact with air and/or water. Some can easily be evaporated while others quickly decompose in heat (sugars, proteins, fats, …). In order to deal with all sorts of samples, essentially from stone to tissue, from metal to gas, and from neutral molecules to ionic species, mass spectrometry utilizes a plethora of ionization methods.
For the determination of elements contained in inorganic materials such as metals, metal oxides, minerals etc. one needs techniques capable of splitting the sample material into its atomic constituents. These are extremely harsh ionization methods often involving very high temperature and electric discharges of various kinds, e.g., thermal ionization (TI), spark sources (SS), inductively-coupled plasmas (ICP), and glow discharges (GD). While these methods reveal the atomic composition of the sample material, they do not anymore provide information on chemical bonding.
For the analysis of small molecules, the most frequently employed techniques are electron ionization (EI), chemical ionization (CI), and field ionization (FI). These methods are getting softer and softer from EI to CI to FI. FI can yield spectra that essentially show the intact molecular ion only. Whatever the softness of the actual ionization process may be – the above three methods require the sample to be evaporated prior to ionization.
Finally, there are some extremely soft ionization methods that combine the process of transporting molecules into the gas phase and formation of ions thereof into one process. Such methods are therefore termed desorption/ionization methods. They allow mass spectral analysis of very large, highly polar, and otherwise difficult to handle compounds.
In the 1980s fast atom bombardment (FAB) and field desorption (FD) were the dominating desorption/ionization methods. Today, electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI) are in use. Especially the last two techniques meant a breakthrough for mass spectrometry in the field of biochemical and biomedical research. ESI and MALDI enabled the analysis of proteins and peptides, of oligosaccharides and lipids, nucleic acids etc. This allows us to decipher the mechanisms metabolism, of infection, of interaction of pharmaceuticals with organs etc. on the molecular level. Often, mass spectrometry is used in combination with separation techniques like gas chromatography (GC-MS) or liquid chromatography (LC-MS).
There is still enormous progress being made. More recent methods of ambient desorption/ionization like desorption electrospray ionization (DESI) or direct analysis in real time (DART) allow to detect samples from surfaces by simply exposing these surfaces to the ionization zone of the ion source at the open atmosphere. This enables quick and facile quality control as well as detection of drugs of abuse, explosives or pesticides.
Successful mass spectral analysis of any sample requires the careful selection of an ionization method and other steps of the procedure. This demands that the mass spectrometrists in charge has some knowledge about the capabilities as well as the limitations of these various methods and instrumentation in general.